Regulatory Roll Call: Stronger Organic Standards, Farm Bill Updates, And How To Disclose Non-GMO Foods
As we look into the Nosh crystal ball and attempt to make sense of what to expect in 2024, we called up a few regulatory experts to run through what they are watching this year.
Sam Jockel is partner at law firm Alston and Bird and previously served as counsel at both the USDA and FDA and Kristi Boswell, also counsel at Alston and Bird, is a former Senior Adviser to the Secretary of Agriculture. Here’s a look at what they are watching for this year:

The Strengthening Organic Enforcement Rule, which Jockel characterized as one of the most significant updates to organic regulations in the past decade, will go into effect in March. The finalized rule aims to address concerns about the integrity of the USDA Organic seal and the potential for fraud in the global organic market.
Numerous changes will hit up and down organic products’ supply chain. For example, producers who import organic ingredients or products will now be required to obtain electronic import certificates. Organic product processors and handlers will also now be required to hold the certification through the USDA, but retailers and restaurants will be allowed certain exceptions.
Jockel believes these changes will arm the USDA with greater control over the organic supply chain. “I don’t know exactly how that’s going to play out, but it will certainly give them more authority to go after entities throughout the supply chain,” Jockel said.
There will also be stricter guidelines around what substances can be used at the crop level– meaning plant protectors, herbicides, pesticides – and allow producers to maintain a USDA organic seal, Boswell added.
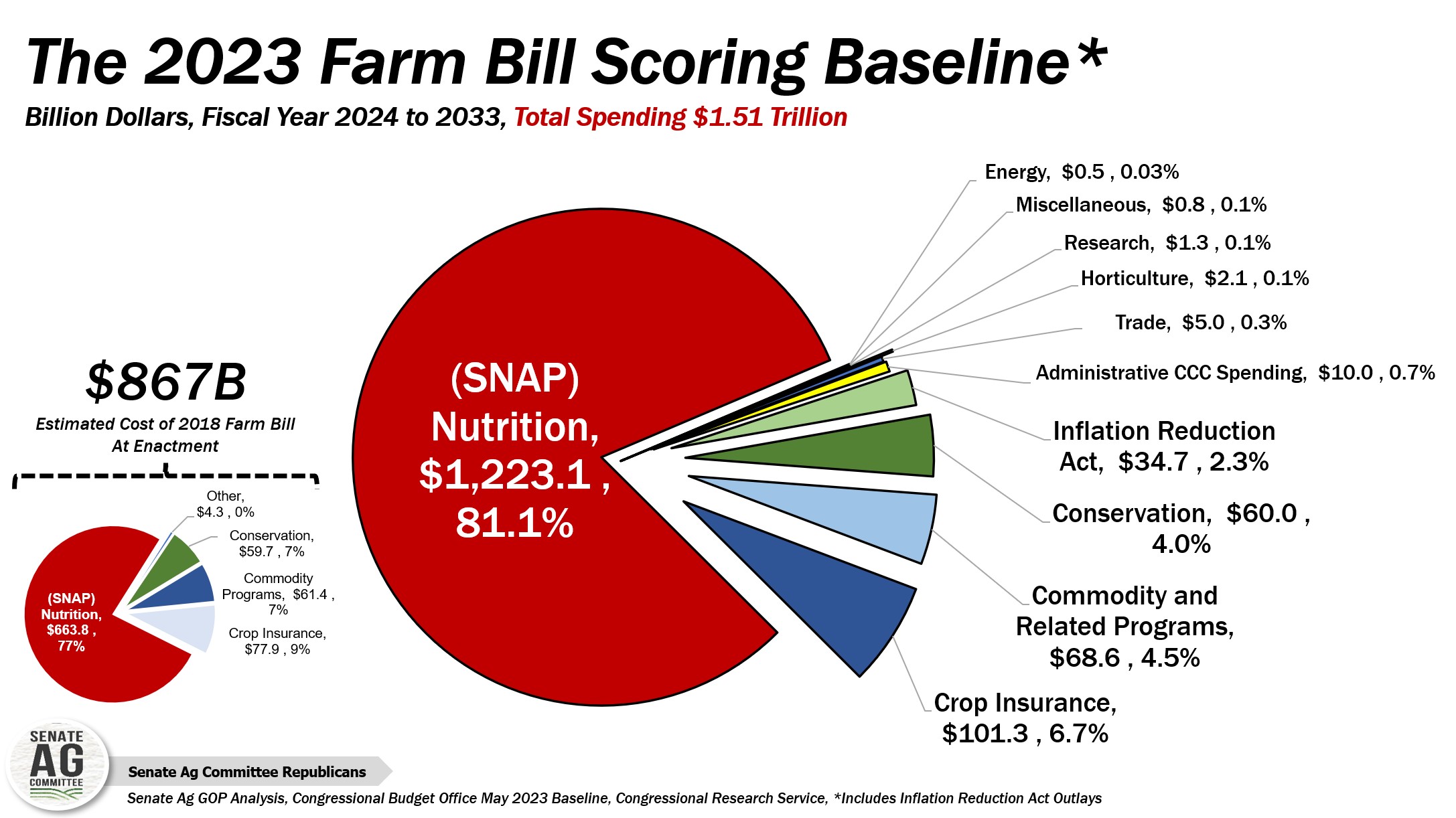
What’s Up With The Farm Bill? Well, according to Boswell, all committees involved in drafting the new bill are in agreement on two things: they want to see a new farm bill, and the uncertainty around the farm bill harms the food and agriculture sectors. These groups often have to make business decisions multiple years ahead of their realized impact or implementation so not knowing what programs or funding will come from the bill hinders their ability to make informed choices.
Thanks to encouraging recent comments from House Agriculture Committee Chairman Glenn Thompson (R-PA), Boswell believes there is a potential for a draft of the bill to come as soon as March. She emphasized that if the bill comes closer to the end of its one-year extension (expiring September 2024) then any chance of it being passed will come in around the 2024 presidential election, making matters even more difficult.
As for what could come from the new document – Jockel stated that any outcome remains uncertain at this point but noted that hemp industry stakeholders have been lobbying for more clarity on the use of CBD and other hemp derivatives in food products.
A coalition of hemp industry organizations recently outlined a slate of changes for federal regulators that would, among other directives, push the FDA to regulate CBD-containing CPG products under the Federal Food, Drug and Cosmetic Act. Separately, the marijuana industry has urged lawmakers to create a stricter definition of hemp and effectively ban delta-8 THC hemp products.
If more clarity does come with the new bill, any impact on the industry is still ways away. Once the bill is signed into law it will move to the Agricultural Marketing Service (AMS), Boswell explained. The department then analyzes the bill and decides if further rule making is required, which could take up to a year considering the public commentary and implementation processes. That puts any final regulation to come by 2025 at the earliest.

Jockel and Boswell expect to see judgment on lingering issues surrounding Bioengineered or non-GMO Food Disclosures this year. The Bioengineered Food Disclosures Act, which passed in 2016 and saw finalized guidance go into effect in 2022, sparked immediate debate as it permits food producers to use electronic disclosures, most often a QR code, for mandatory disclosures.
Retailers including Natural Grocer, in addition to nonprofits and consumer groups sued the USDA over the rule claiming that a QR code would be insufficient. This is the first time digital links have been allowed and can serve as a substitute for on pack claims or symbols.
While that litigation is ongoing, the USDA has taken little to no enforcement action on the rule, Jockel said. He believes this may be because the relevance of non-GMO to consumers has faded a bit in consumer minds and moved on to other issues like environmental contaminants or the safety of certain food ingredients – “maybe it was the flavor of the day.”
















